Confusion Over Drug Efficacy & Potency
DRUG EFFICACY & DRUG POTENCY
- Both of these are
like TWO DIFFERENT beds in a single room!!
- Often these two
terms ‘Drug Potency’ & ‘Drug Efficacy’ are used relatively, but these are
not synonymous & refer to different characteristics of the drug
- So, this is the
article from where you may clearly understand the difference between these two
terms which we mentioned earlier as two different beds in a single room!
1. DRUG POTENCY:
- Amount of Drug Needed to produce maximum effect which is done by the Drug-Receptor Interaction.
- It is the ability/affinity of drug to bind receptors.
- Amount of drug is
inversely proportional to potency that means lesser the amount of drug required
to produce maximum effect, more will be the potency of that drug.
- It is also known
as Drug Strength.
- Potency refers to the concentration OR dose of a
drug required to produce 50% of that drug’s maximal effect.
2.DRUG EFFICACY [ EMAX ]:
- Efficacy is the concept of a drug after interaction with receptor.
- Hence it is an Ability of Drug to produce effect.
- This is the ability to initiate conformational changes in receptors which leads to certain effects. Efficacy is the concept of a drug after interaction with receptor.
- In other words, it is the capacity of a drug to produce a maximum response.
- Hence, it can also be termed as Maximal Efficacy [ EMAX ]. Maximal Efficacy of a drug assumes that all receptors are occupied by drug & if more drugs are added, no further additive response will be observed.
- Collectively, it can define as the ability of a drug to elicit a physiological response when it interacts with receptor.
- Efficacy is depending on:
1. Number of Drug-Receptor Complexes are formed.
2. Efficiency of the coupling of receptor activation to cellular response.
Just_Touch_me to get brief about Difference between Drug Potency & Drug Efficacy.
Both of these Terms are Mathematically Understandable from DOSE
RESPONSE CURVE.
DOSE RESPONSE CURVE:
- Two aspects viz;
(I) Dose-Plasma Concentration Relationship
(II) Plasma Concentration-Response Relationship
- Intensity of Response Increases with
Increase in Dose / more Precisely Increase in Concentration at Receptor).
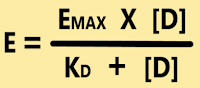
- Drug-Receptor Interaction obeys Law of
Mass Action, which is illustrated by the formula;
- Where, E is Observed Effect at a Dose [D]
of drug; EMAX is Maximal Response; KD is Dissociation Constant of Drug-Receptor
Complex.
- Dose-Response Curve is a Rectangular
Hyperbola Logarithmic Dose-Response Curve is Sigmoid Drug Potency &
Efficacy can be determined by Drug Response Curve.
- Potency & Efficacy of Drug can be determined by Dose
Response Curve as shown below:
Drug B is Less Potent but Equally Efficacious as Drug A
Drug C is Less Potent and Less Efficacious than Drug A
Drug D is More Potent than Drugs A, B, & C, but Less Efficacious than Drugs A & B,
& Equally Efficacious as Drug C
- Log Dose-Response Curves [DRC] IS Plotted because it has several
advantages like;
(I) Wide Range
of Drug Doses can be Easily Displayed on a Graph
(II) Comparison
Between Agonists & Study of Antagonists becomes Easier
Dose-response curves are used to derive Dose Estimates of
chemical substances.
DOSE ESTIMATES:
There are Three important Dose Estimating classical terms are
there which are:
1.Lethal Doses [LDs]
2.Effective Doses [EDs]
3.Toxic Doses [TDs]
1.Lethal Doses [LDs]:
- Initially, LD50 (Lethal Dose 50%)
has been a common dose estimate for acute toxicity.
- LD50 is statistically derived
maximum dose at which 50% of the group of organisms (rat, mouse, or other
species) would be expected to die.
- LD50 testing is no longer the
recommended method for assessing toxicity because of the ethics of using large
numbers of animals, the variability of responses in animals and humans &
the use of mortality as the only endpoint.
- Regulatory agencies use LD50 only if
it is justified by scientific necessity & ethical considerations.
- Concentrations / Lethal Dose 0%
(LD0) represents the dose at which no individuals are expected to die. This is
just below the threshold for lethality.
- Concentrations / Lethal Dose 10%
(LD10) represents the dose at which 10% of the individuals will die.
- Lethal Concentration 50% (LC50) refers
for inhalation toxicity, air concentrations are used for exposure values. The
LC50 refers to the calculated concentration of a gas lethal to 50% of a group.
Very occasionally LC0 and LC10 are also used.
2.Effective Doses [EDs]:
- Effective Doses (EDs) are used to indicate the effectiveness
of a substance.
- Normally, effective dose refers to a beneficial effect such
as relief of pain.
- It may also stand for a harmful effect such as paralysis.
- Thus, the specific endpoint must be indicated.
- The usual terms are: ED0, ED10, ED50, ED90.
3.Toxic Doses (TDs):
- Toxic Doses (TDs) are used to indicate doses that cause
adverse toxic effects.
- The usual dose estimates include: TD0, TD10, TD50, TD90. [TD50 is Dose required to produce a toxic effect i 50% of population].
Determining the Pharmaceutical Relative Safety:
- Toxicologists, pharmacologists & others use effective and
toxic dose levels to determine the relative safety of pharmaceuticals.
THERAPEUTIC INDEX:
- Quantitative measurement of relative safety of drug.
- Therapeutic Index (TI) is used to compare the therapeutically
effective dose to the toxic dose of a pharmaceutical agent.
- TI is a statement of relative safety of a drug.
- It is the ratio of the dose that produces toxicity to the dose
needed to produce the desired therapeutic response.
- The common method used to derive the TI is to use the 50%
dose-response points, including TD50 & ED50.
- Que. If the TD50 is 100 & the ED50 is 20 mg, then TI
would be?
- More the Safety if more is TI [TI directly Proportional to
Safety]
- In Low TI i.e., Low Safety Margin, even the smaller increment
in the dose would produce toxic effects.
- Like that, In High TI i.e., High Safety Margin, more
reliability of the dose is possible since it would not produce toxic effects
after smaller increment in dose.
- Bioavailability of a drug should be done at Low TI.
- However, the use of the ED50 & TD50 doses to derive the
TI may be misleading about a drug's safety, depending on the slope of the
dose-response curves for therapeutic & toxic effects.
- To overcome this demerit, toxicologists often use another term to denote the safety of a drug i.e., Margin of Safety [MOS].
.
.
.
.
.
Stay tune...
Stay Safe...







Comments
Post a Comment
If you have any query or If you like the post,Please let me know.