REMDESIVIR - A BURNING TOPIC
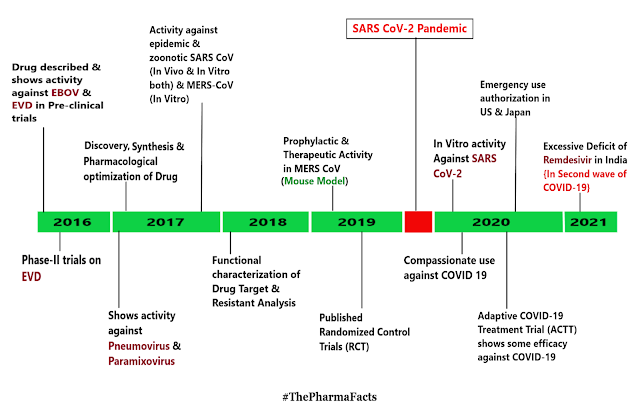
Patients all over world are facing tremendous health care hazards that are caused by the Severe Acute Respiratory Distress Syndrome Coronavirus 2 (SARS-CoV-2) pandemic. Remdesivir {First described in 2016} is the first approved treatment for severe coronavirus disease 2019 (COVID-19). It is a "Novel Nucleoside Analog" with a broad antiviral activity spectrum among RNA viruses, including Ebolavirus (EBOV) and Respiratory Pathogens like the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2. In vivo, Remdesivir showed therapeutic as well as prophylactic effects in animal models of EBOV, MERS-CoV, SARS-CoV, and SARS-CoV-2 infection. However, the substance failed to some extent in a clinical trial on ebola virus disease (EVD), where it was inferior to investigational monoclonal antibodies in an interim analysis. As there was no placebo control in this study, no conclusions on its efficacy in EVD can be made. In contrast, data from placebo-controlled trials show beneficial effects for patients with COVID-19. Remdesivir reduces the time to recovery of hospitalized patients who require supplemental oxygen and may have a positive impact on mortality outrage, while having a favorable safety profile. Although this is an important milestone in the fight against COVID-19, approval of this drug will not be sufficient to solve the public health issues caused by the ongoing pandemic. Further scientific efforts are needed to evaluate the full potential of nucleoside analogs ( like Remdesivir ) as treatment or prophylaxis of viral respiratory infections and to develop effective antivirals that are effective & orally bioavailable.
It was December 2019,a novel coronavirus (nCoV), Severe Acute Respiratory Distress Syndrome CoronaVirus-2 (SARS-CoV-2), emerged in central China (Wuhan). Like in SARS-CoV, infections with the closely related SARS-CoV-2 cause a respiratory disease that can progress to viral Pneumonia and Acute Respiratory Distress Syndrome (ARDS) . Because of its onset in December 2019, the associated disease was called coronavirus disease 2019 (COVID-19).
Till today this pandemic causes several deaths worldwide & hitting every part of world so badly🌎. In the spectra of an uncontrolled expansion and the steadily increasing COVID-19 fatalities in summer 2021 (As per Indian Time), huge efforts were put into the identification of effective antiviral agents against COVID-19. Nucleoside/nucleotide analogs are one of the most promising antiviral drug classes in general, and significant drug discoveries emerged from this class that today form the basis for treatments against infections with several herpesviruses, Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV). Remdesivir is a prodrug of a nucleoside analog with direct antiviral activity against several single-stranded RNA viruses, including SARS-CoV and MERS-CoV. The first cell-based studies of Remdesivir also showed antiviral activity against the novel SARS-CoV-2. In the absence of any effective treatment options against COVID-19, Remdesivir has been applied under "compassionate use" & not 100% efficient & complimentary drug. Recently, a randomized placebo-controlled clinical trial showed that Remdesivir reduces the time to recovery in patients with COVID-19, leading to an Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) only 2 days after the first press release from the National Institute of Allergy and Infectious Diseases (NIAID). On 3 July, the European Medicines Agency (EMA) granted a conditional marketing authorization for Remdesivir, now being the first approved antiviral treatment against COVID-19.Growth of Remdesivir is shown below.
What is Remdesivir?
Being a Pharmacist, we study all category drugs from normal sneezing coughing to highly dangerous cancer, but nowadays we all are suffering from covid-19 pandemic and this is the second wave of Corona virus and its severity and mortality rate increases more day by day. Now as the Covid-19 cases set a new benchmark daily.
This drug is a highly demanded drug across india. Cases are touching new high daily so ultimately demand is also high but its supply is down so across. We heard in many cities reports of fake injections of remdesivir and massive black marketing of the vials.
So Many Questions are arises in front of you guys as a pharmacist. What is this remdesivir & which is its class? Is it really significant over Covid-19? Who permits this drug over covid-19 treatment ? Which side effects may be shown if we take it as chronic treatment ? Which Mechanism of Action it shows ? Like that so we will discuss here pointwise.
It is Novel Antiviral drug belonging to Nucleotides analog. The Chemical structure of Remdesivir shown below, directs that structurally Remdesivir resembles Nucleotide with Phosphate derivative where one part is Nucleoside core & another is Prodrug Component.It is Antiviral Family Drug.
Mechanism of Action of Remdesivir :
Remdesivir is a Monophosphoramidate Nucleoside Prodrug that undergoes intracellular metabolic conversion to its active metabolite Nucleoside Triphosphate (NTP) as shown in the diagram below.
As described for several other direct-acting antivirals, the active metabolite of Remdesivir Triphosphate [Remdesivir-TP] subsequently targets the machinery responsible for the replication of the viral RNA genome, a highly conserved element of the viral life cycle. Nucleoside analogs are synthetic compounds that work by competition with endogenous natural nucleoside pools for incorporation into replicating viral RNA. While these compounds mimic their physiological counterparts, the incorporation of the analog molecule disrupts subsequent molecular processes. The drug target and the exact processes that lead to the inhibition of viral replication have been studied extensively in ebolavirus.
The suggested drug target, the EBOV RNA-dependent RNA polymerase (RdRp) complex, was only recently biochemically purified, which allowed for in-depth molecular analyses. Viral RdRp is the target protein for the active metabolite remdesivir-TP. Remdesivir-TP acts as the substrate for RdRp where it competes with ATP for incorporation into new strands. Inhibition of EBOV or Covid-19 RdRp most probably results from delayed chain termination, a mechanism that is known from approved antivirals against human immunodeficiency virus type 1 (HIV-1) and HBV (Hepatitis). Most importantly, the activity of human RNA polymerase is not inhibited in the presence of Remdesivir-TP.
Intracellular activation of Remdesivir and inhibition of coronavirus replication --- Way through the cell membrane by Remdesivir is facilitated by the prodrug component attached to the nucleoside core. Upon entering the target cell, the pronucleotide undergoes further phosphorylation steps to become the active Triphosphate metabolite that effectively inhibits viral RNA replication.
Delayed chain termination is caused by the following processes:
(i) Disintegration of nucleoside triphosphate (NTP) into replicating RNA by RdRp (ii) Prevention of further chain elongation after NTP along with 3 additional nucleosides and premature termination of RNA synthesis.
Side Effects :
In the Ebola trial, researchers noted side effects of remdesivir (RDV) that included:
-Increased liver enzyme levels that may indicate possible liver damage
-Nausea
-Vomiting
Dosage :
The dosing regimen understudy in the U.S. for RDV is as follows:
-An initial one-time dose of 200mg
-Followed by 100mg per day for 10 days
-Remdesivir is administered intravenously (IV) by injection.
Contraindications :
(In Pregnant women or Breastfeeding women)
In rats and monkeys, RDV affected kidney development in fetuses.
It is unknown if RDV passes into breast milk. Consult your medical practitioner before breastfeeding.
Why Remdesivir Is Not Ultimate Drug Against Covid-19?
In SARS-CoV and MERS-CoV, Remdesivir-TP interferes with the nonstructural RNA binding protein i.e. nsp12 polymerase, which is a multisubunit RNA synthesis complex of viral nonstructural proteins (nsp) produced as cleavage products of viral polyproteins. As nsp12 is highly conserved across the coronavirus family, it is most likely that the mechanism of action of Remdesivir does not differ significantly among CoVs.
Like in EBOV, Remdesivir-TP efficiently inhibits the replication of SARS-CoV and MERS-CoV by causing delayed chain termination when being incorporated into the replicating RNA. A recent biochemical analysis revealed that in SARS-CoV-2, Remdesivir-TP causes the termination of RNA synthesis at three positions after the position where it is incorporated. This mechanism was nearly identical in RdRp of SARS-CoV and MERS-CoV. The premature termination of RNA synthesis ultimately restricts further transcriptional and translational processes needed for the generation of new virions (as shown in dia-MOA).
Thus Remdesivir isn't the utmost medication to treat Covid-19 patients but can be used as the first line of defense against it. Using Remdesivir in combination with some still-found or yet undiscovered drug that could block nsp12 more efficiently & in a broad continuous manner (since it is highly conserved across Coronavirus) could turn out to be a more sure-fire strategy than Remdesivir alone.
SO TRY TO IMPROVE YOUR NATURALLY ADAPTED IMMUNITY BY CONSUMING SEVERAL CITRUS FRUITS,ALMONDS,SPINACH,GARLIC ALONG WITH HEALTHY LIFE CYCLE !




Comments
Post a Comment
If you have any query or If you like the post,Please let me know.