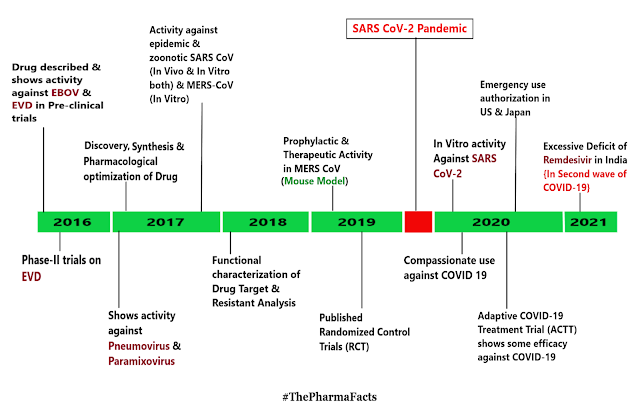
REMDESIVIR - A BURNING TOPIC
Patients all over world are facing tremendous health care hazards that are caused by the Severe Acute Respiratory Distress Syndrome Coronavirus 2 (SARS-CoV-2) pandemic. Remdesivir {First described in 2016} is the first approved treatment for severe coronavirus disease 2019 (COVID-19). It is a " Novel Nucleoside Analog " with a broad antiviral activity spectrum among RNA viruses, including Ebolavirus (EBOV) and Respiratory Pathogens like the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2. In vivo, Remdesivir showed therapeutic as well as prophylactic effects in animal models of EBOV, MERS-CoV, SARS-CoV, and SARS-CoV-2 infection. However, the substance failed to some extent in a clinical trial on ebola virus disease (EVD), where it was inferior to investigational monoclonal antibodies in an interim analysis. As there was no placebo control in this study, no conclusions on its efficacy in EVD can be ...



Comments
Post a Comment
If you have any query or If you like the post,Please let me know.