Afghanistan is the first country to recognize Indian Pharmacopoeia.
Indian Pharmacopoeia is an official book published by the Ministry of health and family welfare; which includes drug monographs, standard operating procedures & various guidelines from manufacturing to storage. In India, every pharmaceutical industries follow this book as officially.lets come to our headline
The Indian Pharmacopeia (IP) has been perceived officially by the National Department of Regulation of Medicines and Health Products of the Ministry of Public Health of the Islamic Republic of Afghanistan. Based on their requirement, they will use our reputed pharmacopeia for improvement in the laboratory of medicine and healthcare quality products. With this step, Afghanistan has become the first nation to refer to Indian pharmacopeia as their official choice.
The safety, quality, and stability of the medications are significant from a medical care point of view. Indian Pharmacopeia Commission (IPC) as Indian pharmacopeia will help to ensure the quality & Safety of medicinal products. According to, the Second Schedule of the Drugs and Cosmetics Act, IP is assigned as the official book of guidelines for drugs imported/or homemade available to be purchased, stock, or display available to be purchased or Distribution in India.
The IP Commission's main goal is to enhance the public health in India by bringing out definitive and authoritatively acknowledged norms for the nature of medications including dynamic drug fixings, excipients, and dose structures, utilized by Physician Professionals, hospital inpatients-outpatients & consumers. This is accomplished by building up the norms for prescriptions and supporting their execution.
Furthermore, IPC likewise creates IP Reference Substances (IPRS) that go about as unique mark for the ID of an article under test and its standards as recommended in the IP monographs. Norms recommended in the IP are legitimate and are authorized by the administrative experts for quality control of meds in India.

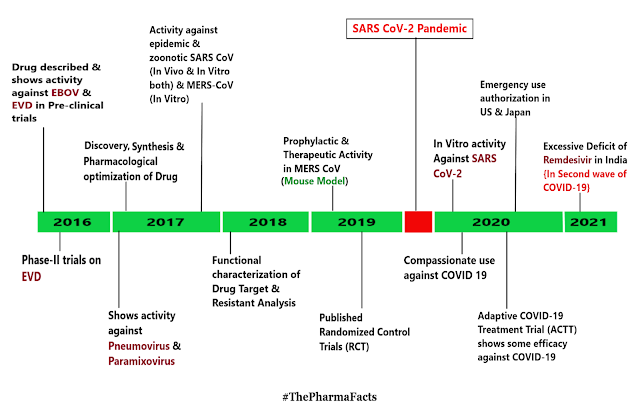

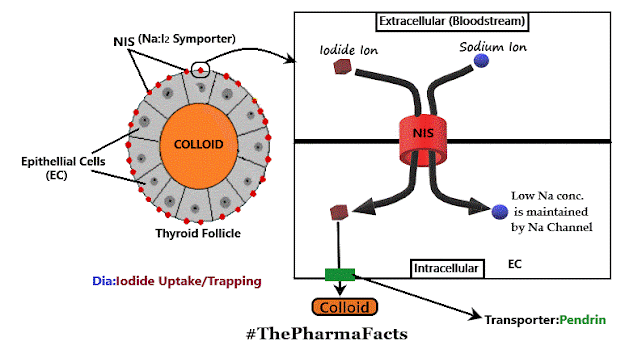

Comments
Post a Comment
If you have any query or If you like the post,Please let me know.